Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
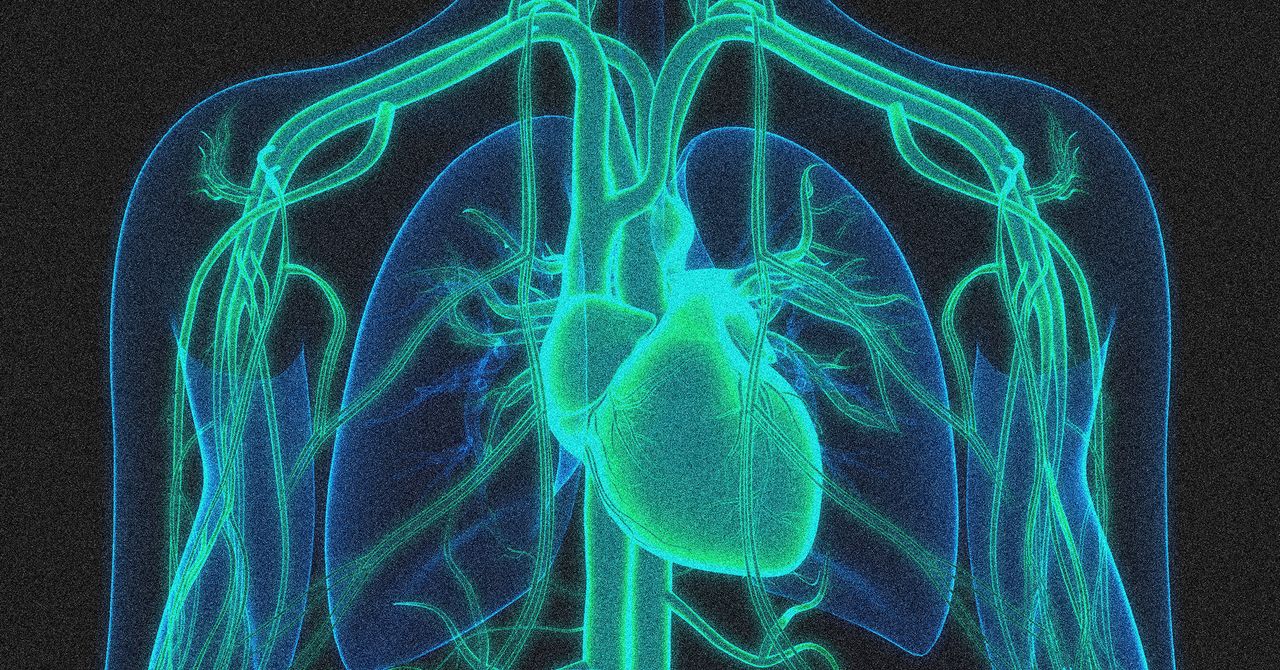
In step Towards wider use of gene editing, a treatment that is being used CRISPR It has been successful in lowering high cholesterol levels in a small number of people.
In a trial conducted by the Swiss biotech company Crispr Therapeutics, 15 participants received a one-time injection aimed at turning off a gene in the liver called Angptl3. Although rare, some people are born with a mutation in this gene that protects against heart disease without any obvious negative consequences.
The highest dose tested in the trial reduced LDL cholesterol and triglycerides by an average of 50 percent within two weeks after treatment. The effects lasted for at least 60 days, the duration of the trial. The findings were presented today at the American Heart Association’s annual meeting and published in New England Journal of Medicine.
The Nobel Prize-winning Crispr technology has been mostly used Treatment of rare diseasesBut these latest results, while early, add to the evidence that the DNA editing tool could be used to treat common conditions as well.
“This is potentially one of the biggest moments in the arc of Crispr development in medicine,” Samarth Kulkarni, CEO of Crispr Therapeutics, tells WIRED. The company behind the only approved gene editing treatment on the market, KasjiviWhich treats sickle cell anemia and beta thalassemia.
American Heart Association Estimates About a quarter of adults in the United States have elevated LDL levels. A similar number have high triglycerides. LDL cholesterol is the waxy substance in the blood that can clog and harden the arteries over time. Meanwhile, triglycerides are the most common type of fat found in the body. High levels of both increase the risk of heart attack and stroke.
The phase 1 trial was conducted in the United Kingdom, Australia and New Zealand between June 2024 and August 2025. Participants were aged 31 to 68 years and had uncontrolled levels of LDL cholesterol and triglycerides. The trial tested five different doses of CRISPR infusion, which took about two and a half hours on average.
“These are very sick people,” says Steven Nissen, first author and academic director of the Cardiovascular and Thoracic Institute at Cleveland Clinic, who independently confirmed the trial results. “The tragedy of this disease is not only that people die young, but some of them will have a heart attack, and their lives will never be the same again. They don’t go back to work, and they develop heart failure.”
One trial participant, a 51-year-old man, died six months after receiving the lowest dose of the treatment, which was not associated with lower cholesterol and triglycerides. The death was related to his heart disease, not the experimental CRISPR treatment. The man had a rare genetic form of high cholesterol and had previously undergone several procedures to improve blood flow to his heart.